Department of Experimental Medicine I
We investigate tumor progression from initiation of precursor lesions towards invasion and metastasis. Based on understanding the underlying molecular mechanisms, our aim is to develop novel therapeutic concepts against these processes. We integrate basic as well as translational and clinically relevant research aspects and focus on gastrointestinal cancer, but also include other cancer types for validation of results.
The Chair of Experimental Medicine 1 is localized at the Nikolaus-Fiebiger-Center for Molecular Medicine of the Friedrich-Alexander-Universität (FAU) Erlangen-Nürnberg. The FAU was founded in 1743 and is one of the biggest universities in Germany with more than 40,000 students (https://www.fau.de/).
Erlangen is located in northern Bavaria in close proximity to Nuremberg (25 km), the world heritage site Bamberg (30 km) and Munich (180 km).
The closest airports are Albrecht-Duerer-Airport Nuremberg (15 km) and Franz-Josef-Strauss Airport Munich (170 km).
Schwerpunkt
Der Schwerpunkt der Forschungsarbeiten liegt auf der Entstehung und Ausbreitung von Krebserkrankungen, insbesondere auf molekularen Mechanismen der Tumorinvasion und Metastasierung. Das Ziel ist die Entwicklung neuer Therapiekonzepte zur Bekämpfung dieser Prozesse. Dabei integriert die Arbeitsgruppe zellbiologische, molekularbiologische und genetische Methoden, Zellkultur- und Tiermodelle, sowie Analysen von humanen Tumorproben und Patientendaten. Im Fokus stehen gastrointestinale Tumorerkrankungen (v.a. Pankreas- und Darmkrebs), es werden aber auch andere Tumoren (z.B. Brust- und Lungenkrebs) in die Forschung mit einbezogen.
Forschungskonzept
Unser Forschungskonzept basiert auf klinisch relevanten Fragestellungen, insbesondere auf den wichtigsten Problemen der heutigen Krebsmedizin. Dazu zählen die Entwicklung einer Therapie-Resistenz, z.B. gegen etablierte Chemotherapeutika bei hämatologischen und soliden Neoplasien, sowie die Metastasierung solider Tumore. Dementsprechend sind die Überwindung der Radio-Chemoresistenz sowie die Entwicklung neuer, gezielter Therapien sowohl gegen Tumor-Dissemination und Metastasierung die zentralen Herausforderungen der translationalen Krebsforschung, denen wir uns stellen. Wir konnten zeigen, dass die besondere Fähigkeit von Krebszellen, sich an unterschiedlichste Bedingungen und Anforderungen anzupassen, einen wesentliche Triebkraft der Progression bis zu einer therapie-resistenten, metastatischen Erkrankung ist. Diese Fähigkeit wird als aberrante, zelluläre Plastizität bezeichnet, die in vielen Tumoren ermöglicht die transiente Aktivierung des embryonalen EMT-Programms ermöglicht wird. Eine entscheidende Rolle kommt hier den EMT-aktivierenden Transkriptionsfaktoren (EMT-TFs), wie dem Faktor ZEB1, zu. So konnten wir z.B. zeigen, dass der zellulären Plastizität ein von uns identifizierter molekularer Motor – der ZEB1/miR-200 Feedback-Loop – zugrunde liegt.
Ziele
Aktuelle Arbeiten befassen sich mit der Rolle der EMT/zellulären Plastizität bei Krebs (und anderen Krankheiten) und erforschen sie als Ziel therapeutischer Interventionen. In verschiedenen Projekten untersuchen wir:
- die Rolle der EMT/TFs bei Krebs-Entstehung, Metastasierung und Therapieresistenz,
- das Mikroenvironment als Modulator der zellulären Plastizität bei Krebs,
- nicht-redundante und nicht-EMT-bezogene Funktionen der EMT-TFs,
- spezifische epigenetische Mechanismen der verschiedenen EMT-TFs,
- neuartige Mechanismen, die der zellulären Plastizität zugrunde liegen,
- und erforschen die translationale und klinische Relevanz unserer Erkenntnisse durch die Entwicklung neuartiger diagnostischer Werkzeuge und Behandlungsstrategien für humane Krebserkrankungen.
Zur Beantwortung unserer Forschungsfragen setzen wir modernste Technologien ein, verfolgen molekulare, epigenetische und genetische in vitro Ansätze, verwenden Zell- und Tiermodelle (z.B. genetische Maus-Tumormodelle und Tumororganoide). Wir validieren die Ergebnisse an humanem Tumormaterial und Patientendaten, u.a. durch den Einsatz bioinformatischer Analysen.
Detaillierte Informationen rund um das Studium der Molekularen Medizin erhalten Sie auf den Seiten der Medizinischen Fakultät der FAU.
Informationen zum Bachelorstudiengang
Informationen zum Masterstudiengang (nur in englischer Sprache verfügbar)
Kursmaterialien und Unterlagen zum Download finden Sie im E-Learning Portal der FAU ![]()

Das Nikolaus-Fiebiger-Zentrum (NFZ) für Molekulare Medizin ist eine Forschungseinrichtung der Medizinischen Fakultät der Friedrich Alexander Universität (FAU) Erlangen-Nürnberg. In diesem Zentrum sind zwei Lehrstühle für Experimentelle Medizin (Exp Med 1 – Molekulare Pathogeneseforschung und Exp Med 2 – Molekulare Tumorforschung), die Abteilungen für Molekulare Immunologie und Immun-Genetik, sowie eine Reihe weiterer Forschungs-Nachwuchsgruppen des Interdisziplinären Zentrums für klinische Forschung (IZKF) untergebracht. Die Intention dieses Forschungszentrums ist, die biomedizinische Forschung der Medizinischen Fakultät zu stärken, indem die Kooperationen zwischen Grundlagen- und klinischen Forschern angeregt werden. Auch soll jungen klinischen Forschern die Möglichkeit gegeben werden,kompetitive biomedizinische Forschungsprojekte unter der Infrastruktur eines modernen Forschungsinstituts zu verfolgen.Das Nikolaus-Fiebiger-Zentrum ist hervorragend für zell- und molekularbiologische Forschung ausgestattet, u.a. verfügt es über zentrale Facilities für Cell Sorting, konfokale Laser-Mikroskopie und Tierhaltung. Es bietet eine Vielzahl von Seminaren zu zellbiologischen, onkologischen, immunologischen und biochemischen Themen. Die Lehrstühle, Abteilungen und Arbeitsgruppen des NFZ sind stark in der Lehre für Studierende der Medizin, Molekularmedizin und Biologie engagiert.

Chair:
Cell plasticity at the tumor-host interface:
Principal Investigators:
Postdoctoral fellows:
PhD Students:
Technicians:
Office:
We are interested in basic mechanisms of malignant tumor progression, which are used by all major solid cancers. We focus on gastrointestinal cancers, particularly pancreatic and colorectal cancer, but also include analyses of breast and lung cancer for validation of results.
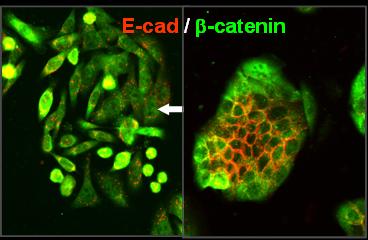
Carcinomas represent the most prevalent malignancies in humans and arise from normal epithelial tissues in a multistep progression from benign precursor lesions. Metastasis, the final step in malignancy, is the major cause of death for cancer patients. Recent models explain selected aspects of the complex tumor progression process: Unrestricted growth, a hallmark of both benign and malignant tumors, can be attributed to cancer stem cells. Generally, a stepwise accumulation of genetic alterations in oncogenes and tumor suppressor genes is considered a driving force for malignancy. The breakdown of epithelial cell homeostasis leading to aggressive cancer progression has been correlated with the loss of epithelial characteristics and the acquisition of a migratory phenotype. This phenomenon, referred to as epithelial to mesenchymal transition (EMT), is considered a crucial event in malignant progression.
EMT not only induces cellular motility, but also confers stemness and survival properties to (cancer) cells. Thus EMT is also participating in the initiation and maintenance of a cancer stem cell phenotype and confers resistance to all treatment modalities in cancer.
Important steps enabling metastasis are reversible, and thus cannot solely be explained by irreversible genetic alterations, indicating the existence of a dynamic component to human tumor progression, and in particular a regulatory role for the tumor environment, which was already demonstrated in many experimental systems. Tumors are not autonomously acting proliferation machines, but are very heterogeneous, both in their morphological and functional aspects. In fact, an individual tumor shows distinct subareas of proliferation and cell cycle arrest, epithelial differentiation and EMT and cell adhesion and dissemination. How are all these different traits orchestrated? It became evident that both common and non-redundant functions of EMT-activating transcription factors (EMT-TFs) play a major role. Notably EMT-TFs can not only activate classical MT-associated properties, but control many additional functions in (cancer) cells.
Cellular plasticity: a driving force for cancer progression and other disease processes
It became evident that cancer cells are highly adaptive to the demanding environmental conditions – a property which can be summarized as aberrant cellular plasticity.
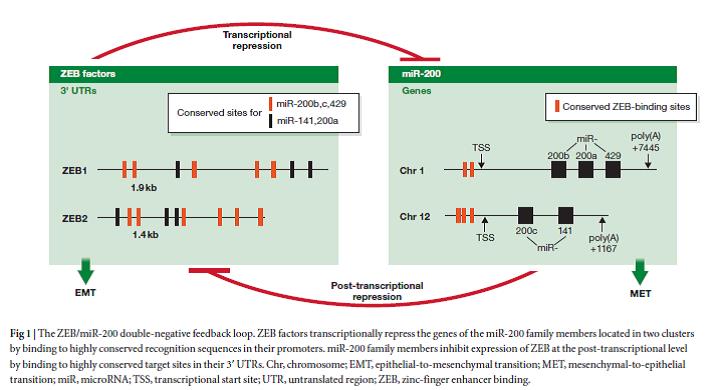
In addition to accumulation of genetic alterations, aberrant cellular plasticity is now considered as a major driving force for cancer progression towards a therapy resistant, metastatic disease, as well as for the pathogenesis of other diseases. Our group has discovered underlying molecular mechanisms controlling cellular plasticity. We have shown that a phenotypic switch between a stemness/EMT state and a differentiated state is exerted by a double-negative feedback loop between the EMT-transcription factor ZEB1 and the miR-200 family of microRNAs, the so-called ZEB1/miR-200 feedback loop.
Research program
Current work addresses the role EMT/cellular plasticity in cancer (and other diseases) and explores it as a target of therapeutic intervention. In various projects we investigate:
- the role of the EMT/TFs in cancer initiation, metastasis and therapy resistance,
- the microenvironment as a modulator of cellular plasticity in cancer,
- non-redundant and non-EMT related functions of EMT-TFs,
- specific epigenetic mechanisms of various EMT-TFs
- novel mechanisms underlying cellular plasticity,
- and explore the translational and clinical relevance of our findings by developing novel diagnostic tools and treatment strategies for human cancer.
To address our research questions, we apply state of the art technologies, persue molecular, epigenetic and genetic in vitro approaches, use cell and animal models (e.g. genetic and grafting mouse tumor models and human/mouse tumor organoids). We validate results on human tumor material and patients’ data by applying bioinformatic tools.
Selected publications (for all publications see PubMed)
The EMT transcription factor ZEB1 governs a fitness-promoting but vulnerable DNA replication stress response. Cell Rep. 2022 Dec 13;41(11):111819. doi: 10.1016/j.celrep.2022.111819. PMID: 36516781
Cytomegalovirus subverts macrophage identity. Cell. 2021 Jul 8;184(14):3774-3793.e25. doi: 10.1016/j.cell.2021.05.009. Epub 2021 Jun 10. PMID: 34115982
The EMT transcription factor ZEB1 blocks osteoblastic differentiation in bone development and osteosarcoma. J Pathol. 2021 Jun;254(2):199-211. doi: 10.1002/path.5659. Epub 2021 May 6. PMID: 33675037
Feldker N*, Ferrazzi F*, Schuhwerk H, Widholz SA, Guenther K, Frisch I, Jakob K, Kleemann J, Riegel D, Bönisch U, Lukassen S, Eccles RL, Schmidl C, Stemmler MP, Brabletz T#, Brabletz S#. Genome-wide cooperation of the EMT-activator ZEB1 with YAP and AP-1 factors in breast cancer. EMBO J, 39(17):e103209 (2020). *equal contribution, #corresponding authors
Stemmler MP, Eccles RL, Brabletz S, Brabletz T (2019). Non-redundant functions of EMT transcription factors. Nat Cell Biol 21:102-112.
Brabletz T, Kalluri R, Nieto MA, Weinberg RA. EMT in Cancer. Nat Rev Cancer. doi: 10.1038/nrc.2017.118 (2018) (invited viewpoint article).
Krebs, A. M., Mitschke, J., Losada, M. L., Schmalhofer, O., Boerries, M., Busch, H., Boettcher, M., Mougiakakos, D., Reichardt, W., Bronsert, P., Brunton, V. G., Pilarsky, C., Winkler, T. H., Brabletz, S., Stemmler, M. P., Brabletz, T. The EMT-activator Zeb1 is a key factor for cell plasticity and promotes metastasis in pancreatic cancer. Nat Cell Biol, 19(5):518-529 (2017)
Lehmann W, Mossmann D, Kleemann J, Mock K, Meisinger C, Brummer T, Herr R, Brabletz S, Stemmler MP, Brabletz T. ZEB1 turns into a transcriptional activator by interacting with YAP1 in aggressive cancer types. Nat Comm, 7:10498 (2016).
Preca BT, Bajdak K, Mock K, Sundararajan V, Pfannstiel J, Maurer J, Wellner U, Hopt UT, Brummer T, Brabletz S, Brabletz T, Stemmler MP (2015). A self-enforcing CD44s/ZEB1 feedback loop maintains EMT and stemness properties in cancer cells. Int J Cancer 137:2566-77
Meidhof M, Brabletz S, Lehmann W, Preca BT, Mock K, Ruh M,Schüler J, Bertold M, Weber A, Burk U, Lübbert M, Puhr M, Culig Z, Wellner U, Bronsert P, Küsters S, Hopt UT, Stemmler MP, Brabletz T. (2015) ZEB1-associated drug resistance in cancer cells is reversed by the class I HDAC-inhibitor mocetinostat. EMBO Mol Med, 7: 831-47 (2015).
Puisieux A, Brabletz T, Caramel J. (2014). Oncogenic roles of EMT-inducing transcription factors. Nat Cell Biol, 16, 488-94.
Brabletz T, Lyden D, Steeg PS, Werb Z. (2013). Roadblocks to translational challenges on metastasis. Nat Med, 19, 1104-9.
Brabletz T. (2012) To differentiate or not – Routes towards metastasis. Nat Rev Cancer, 12, 425-436 (invited perspective article).
Brabletz, S., Bajdak, K., Meidhof, S., Burk, U., Niedermann, G., Firat, E., Wellner, U., Dimmler, A., Faller, G., Schubert, J., Brabletz, T. (2011). The ZEB1/miR-200 feedback loop controls Notch signalling in cancer cells. EMBO J, 30, 770-782.
Wellner U, Schubert J, Burk U, Schmalhofer O, Zhu F, Sonntag A, Waldvogel B, Vannier C, Darling D, zur Hausen A, Brunton VG, Morton J, Sansom O, Schüler J, Stemmler MP, Herzberger C, Hopt U, Keck T, Brabletz S, Brabletz T. (2009). The EMT activator ZEB1 promotes tumorigenicity by repressing stemness-inhibiting microRNAs. Nat Cell Biol, 11, 1487-95.
Burk, U., Schubert, J., Wellner, U., Schmalhofer, O., Vincan, E., Spaderna, S. and Brabletz, T. (2008). A reciprocal repression between ZEB1 and members of the miR-200 family promotes EMT and invasion of cancer cells. EMBO Rep, 9, 582-589.
Spaderna S., O. Schmalhofer, M. Wahlbuhl, A. Dimmler, K.Bauer, A. Sultan, F. Hlubek, A. Jung, D. Strand, A. Eger, T. Kirchner, J. Behrens, Brabletz T. (2008). The transcriptional repressor ZEB1 promotes metastasis and a loss of cellular polarity in cancer. Cancer Res, 68,538-44.
Brabletz, T., Jung, A., Spaderna, S., Hlubek, F. and Kirchner, T. (2005). Opinion: migrating cancer stem cells – an integrated concept of malignant tumor progression. Nat Rev Cancer 5, 744-749.
Brabletz, T., Jung, A., Reu, S., Porzner, M., Hlubek, F., Kunz-Schughart, L.A., Knuechel, R., Kirchner, T. (2001) Variable beta-catenin expression in colorectal cancer indicates a tumor progression driven by the tumor environment. Proc Natl Acad Sci USA, 98, 10356-61.
Recent awards of lab members
2022
- Isabell Armstark & Elisabetta D’Avanzo: Poster Award 10th TEMTIA meeting Paris
2019
- Julia Kleemann: Best Post-Doctoral Presentation Award, 9th EMT International association Meeting, Kumamoto, Japan
- Thomas Brabletz: Betty Hay Oration Award of the EMT International Association (TEMTIA)
- Isabell Frisch: Poster Award CRCL (Cancer Research Center of Lyon) Symposium Lyon
- Thomas Brabletz: Member of Leopoldina (German Academy of Sciences)
2018
- Thomas Brabletz: German Cancer Award
- Kathrin Fuchs: Fritz and Maria Hofmann Award for master’s thesis „The influence of stromal ZEB1 on colorectal cancer characteristics“
- Nora Feldker: Poster Award of the 16th IZKF postgraduate workshop
Prof. Dr. Thomas Brabletz is the Co-spokesperson of the SFB TRR305 that started in 2021.
We are strongly engaged in teaching at different educational levels (Bachelor-, Master-, PhD-students) and disciplines (medicine, molecular medicine, biology). Teaching includes lectures, courses and internships for Bachelor- and Masterstudents. PhD students are integrated in a local graduate school programme. All lab members participate regularly in journal clubs, lab meetings and external seminars of guest speakers.
All information about the study of Molecular Medicine can be found on the pages of the Faculty of Medicine:
Course materials are available for download ![]()
For information about lectures and courses for the current semester, please follow the link to ![]()
The Nikolaus-Fiebiger-Center (NFZ) of Molecular Medicine is a research institution of the Faculty of Medicine at the Friedrich-Alexander  Universität FAU) of Erlangen-Nuernberg. The center harbours two chairs of Experimental Medicine (Molecular Pathogenesis Research and Molecular Oncology), a division of Molecular Immunology, a division of Genetics (Science Faculty) as well as several junior research groups of the Interdisciplinary Clinical Research Center (IZKF). The intention of the research center is to strengthen biomedical research in the Medical School by stimulating cooperations between basic and clinical researchers and giving young clinicians the opportunity to carry out competitive biomedical research projects under the infrastructure of a modern research center.
Universität FAU) of Erlangen-Nuernberg. The center harbours two chairs of Experimental Medicine (Molecular Pathogenesis Research and Molecular Oncology), a division of Molecular Immunology, a division of Genetics (Science Faculty) as well as several junior research groups of the Interdisciplinary Clinical Research Center (IZKF). The intention of the research center is to strengthen biomedical research in the Medical School by stimulating cooperations between basic and clinical researchers and giving young clinicians the opportunity to carry out competitive biomedical research projects under the infrastructure of a modern research center.
The Nikolaus-Fiebiger-Center is well equipped with modern research facilities required for cell and molecular biological research and offers a variety of biochemical, immunological and cell biological seminars, guest lectures and common graduate student seminars. Communication between all scientific and technical staff is greatly facilitated by a modern computer network allowing access to data banks and electronic libraries from each desk. Central equipment such as DNA-sequencing, fluorescence activated cell sorting, confocal laser microscopy, surface plasmon resonance as well as animal facilities are accessible for all scientists and technical personell.
Department of Experimental Medicine I
Nikolaus-Fiebiger-Center for Molecular Medicine
Glückstr. 6
91054 Erlangen
Germany
The Nikolaus-Fiebiger-Center is conveniently located very close to the city center of Erlangen. It is just a short walk from here to the main train station. The closest bus stop is „Hindenburgstraße“, which is a stop along the lines 289, 290 and 252.